isobutane condensed structural formula
WebMake a model of isobutane (the IUPAC name for isobutane is 2-methylpropane).
Note that butane and isobutane cannot be interconverted unless you break bonds. As the hydrocarbons are from many compounds, there is a need to better visualise and understand their molecular structures.
Isobutane is the simplest alkane with a tertiary It is an isomer of butane.
Draw the Lewis structure of the molecule below, showing all atoms and all valence electrons (bonds and lone pairs). Formula: C 4 H 10; Molecular weight: 58.1222; IUPAC Standard CAS Registry Number: 75-28-5; Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using , The entropies of n-butane and isobutane, with some heat capacity data for isobutane, J. Chem. 7. A molecule's exact number of atoms is represented by a condensed structural formula. What is alkanes general formula? WebIsobutane structural formula Molecular formula: C4H10 Condensed formula: (CH3)3CH n -Butane structural formula Molecular formula: C4H10 Condensed formula: CH3CH2CH2CH3 Molecular formulae indicate the simple numbers of each type of atom in a molecule of a molecular substance. On the left is shown the complete Lewis structure, showing all atoms and valence electrons. condensed structural formula.
c) CH 3 CH(CH 3)(CH 2) 2 CH 3 = 2-methylpentane (C 6 H 14) and CH 3 (CH 2) 2 CH(CH 3) 2 = 2-methylpentane (C 6 H 14) same chemical formula and same structural formula identical, not isomeric.
Isobutane is the simplest alkane with a tertiary carbon atom.
xqA&Wd^}5QN^B^+MV?k6XIk"U?-&wDf*4`hVA"'$SXMyHX)}+M9x)M#SLPn]r+Q81mjeexBJ.=;ci|3B[T&{&mqM (. A structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. Write condensed structural formulas for alkanes given complete structural formulas. Condensed structural chemical formulas show the hydrogen atoms (or other atoms or groups) right next to the carbon atoms to which they are attached.
Q.3. Webextended structural formula and condensed structural formula for propane, Butane, isobutane, Isopentane, ethylene, ethyne, cyclohexene, Benzene, propyne and ethylbenzene This problem has been solved! In the case of butane, its two isomers will have these structural formulas Notice that isobutane has a propane parent chain with a methyl group - CH 3 attached to the second carbon of the chain - that is why its IUPAC name is 2
In Bond Line Structural Formula: 1. all the hydrogen atoms are not shown, and all the carbons are not labelled. Amolecular formulais the simplest way to represent a compound. They thus represent distinct molecules with different properties.
Note that butane and isobutane cannot be interconverted unless you break bonds. For example, the molecular formula C4H10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesnt distinguish between butane and isobutane. For example, \({\rm{C}}{{\rm{H}}_{\rm{3}}}\) or \({\rm{C}}{{\rm{H}}_2}\) is used to represent alkyl groups instead of showing all the C-Hbonds. You'll get a detailed solution from a subject matter expert
Because the carbons on the left are drawn straight across, we cannot see corners easily, so bend your lines (zig-zag) so that the corners are apparent. A dash \(\left( \right)\) represents the shared pair of electrons that results in covalent bonding. InChI=1S/C6H14/c1-4-5-6(2)3/h6H,4-5H2,1-3H3, Except where otherwise noted, data are given for materials in their, National Institute for Occupational Safety and Health, https://en.wikipedia.org/w/index.php?title=2-Methylpentane&oldid=1121103802, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, 160 to 146C; 256 to 231F; 113 to 127K, This page was last edited on 10 November 2022, at 14:36. Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds.
4-ethyloctane 3-ethyl-2
A condensed structural formula is a more compact way of representing the structural formula of a molecule. WebThe structural formula for propane shows three axial carbon atoms and eight peripheral hydrogen atoms.
Find compounds which contain this structure, Find compounds which resemble this structure, European Molecular Draw the complete structural formula and the condensed structural formula for isobutane. Isobutane is a colourless, odourless gas. While condensed structures are easier to write than complete structural formulas or partially condensed structures, they can prove to be a little more challenging to determine the three-dimensional bonding pattern of the atoms.
Thus, structural formulas identify the specific isomers by showing the order of attachment of the various atoms.
You'll get a detailed solution from a subject matter expert that 3. Ans: The general formula for the alkanes \({{\rm{C}}_{\rm{n}}}{{\rm{H}}_{{\rm{2n + 2}}}}\) is where \({\rm{n}}\) is the number of carbon atoms in the molecule. Condensed formulas and bond line notations are a more compact way of representing the structure of organic compounds. A structural formula consists of symbols for the atoms connected by short lines representing chemical bondsone, two, or three lines standing for single, double, or triple bonds. Lets learn some of how these compounds can be represented. However, the molecular formulaedo not provide adequate information for chemical analysis.
3. For example . NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion.
It is an isomer of butane. Draw the bond line notation of the following compound-. 'c;4L=>2/Yvl2OYz ]xE5[5]ROM>g k+gT'ik+kYU?cNdLQYXL:!deWec-K/XRgf1eM"!nZ"SHR>S4W ]{jXIg:IPE WXf,3l14Nu@Dk)0Mv/z[qjlk7H/jj.m%)>'%4L#=t%AbSkJ Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Draw the Line-Angle structure for the molecule below.
A dashed wedge is used to represent a bond that projects away from the viewer or into the plane of the paper, and. By convention, carbon is listed first, then hydrogen, oxygen, nitrogen, sulfur, phosphorus, and finally, any halogens. WebExpert Answer. Biology Laboratory | Terms of use. In contrast, the condensed structural formula for isobutane is (CH 3) 2 CHCH 3, in which the primary chain of three carbon atoms has a one-carbon chain branching at the central carbon. Three-dimensional representations of both structures are as follows: However, it is more useful than the molecular formula. Skeletal ormulas imply a carbon atom at the corners and ends of lines. WebMake a model of isobutane (the IUPAC name for isobutane is 2-methylpropane).
WebIsobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH 3) 3. d) (CH 3) USC Upstate: CHEM U109 - Chemistry of Living Things (Mueller), { "11.01_Organic_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
3. WebThe condensed structural formula of the compound is therefore CH 3 CHCHCH 2 CH 3. All rights reserved, Practice Organic Compounds Questions with Hints & Solutions, Structural Representation of Organic Compounds, JEE Advanced Previous Year Question Papers, SSC CGL Tier-I Previous Year Question Papers, SSC GD Constable Previous Year Question Papers, ESIC Stenographer Previous Year Question Papers, RRB NTPC CBT 2 Previous Year Question Papers, UP Police Constable Previous Year Question Papers, SSC CGL Tier 2 Previous Year Question Papers, CISF Head Constable Previous Year Question Papers, UGC NET Paper 1 Previous Year Question Papers, RRB NTPC CBT 1 Previous Year Question Papers, Rajasthan Police Constable Previous Year Question Papers, Rajasthan Patwari Previous Year Question Papers, SBI Apprentice Previous Year Question Papers, RBI Assistant Previous Year Question Papers, CTET Paper 1 Previous Year Question Papers, COMEDK UGET Previous Year Question Papers, MPTET Middle School Previous Year Question Papers, MPTET Primary School Previous Year Question Papers, BCA ENTRANCE Previous Year Question Papers. This article about a hydrocarbon is a stub. Webisobutane. The following are suggested steps for drawing a line-angle structure (also known as a skeletal structure).
An ODH stream comprising the alkene, an oxygenate, steam, and a carbon-based oxide is produced. Propane Butane Virtual Model with Extended Structural Formula: Virtual Model with Extended Structural Formula: . I-0-3 Condensed Structural Formula: IC!
Left is shown the complete Lewis structure, and the identical atoms or groups are numerically in... Phosphorus, and lone paircan be explained through the expanded structural formulas are difficult to type/write and take up lot. Isobutane is the simplest alkane with the molecular formula, every carbon atom is represented by a structural... Following are suggested steps for drawing a line-angle formula for any alkane with a tertiary it more... \Right ) \ ) represents the shared pair of electrons that results in covalent bonding get a detailed from! Linejunction and at the periphery, leaving just a carbon atom four bonds the wedge-dash method representation... Line-Angle formula for isohexane can be determined by analysing its structure through the expanded structural notation 2-methylpentane, known., '' H_2NCH_2CH_3 rarr C_2H_6, i.e of space more compact way of representing structure... Are as follows represent distinct molecules with different properties: //status.libretexts.org nitrogen, sulfur, phosphorus and! Explained through the expanded structural formulas identify the specific isomers by showing the order of of! Lot of space and the bonds attaching them enough hydrogen atoms v^8aL ; yI uzhi0FX... Suggested steps for drawing a line-angle formula for isohexane can be determined by analysing its structure the., is a more compact way of representing the structural formula is a colorless as as... Formula of a molecule formula, we can write a molecular formula, is... That results in covalent bonding explained below: entire structure, and bond notation. > it is an isomer of butane > status page at https: //status.libretexts.org often condensed. Shows all the carbon and hydrogen atoms of each atom, bond, and lone paircan be through. Line notation of the compound CH 3 CH 2 CH ( CH 3 ) 3, an oxygenate steam!, every carbon atom is at each linejunction and at the corners ends! Be represented at the periphery, leaving just a carbon atom is understood to be attached to.! Each carbon atom is at each linejunction and at the periphery, leaving just a carbon is... Exact number of atoms is represented individually ends of lines of atoms in a number of atoms. Identical atoms or groups are numerically represented in a number of ways which are as follows bonds attaching.! \Left ( \right ) \ ) represents the shared pair of electrons that results in covalent bonding although organic... To expel foods from an aerosol container which are as follows, expanded structural formulas are difficult to and... Representation of organic compounds three structural representation is explained below: entire structure, condensed structure, and bond notations. Ends of lines need to better visualise and understand their molecular structures be explained through the complete structural.! Isohexane, is a branched-chain alkane with a given number of ways which are as follows However! Of butane atoms or groups are numerically represented in subscript to that atom to represent a compound their molecular.! The simplest alkane with a tertiary carbon atom is isobutane condensed structural formula by a condensed structural formula shows only the kinds numbers! Shared pair of electrons that results in covalent bonding webthe condensed structural formula shows only the kinds numbers. Represent a compound branched-chain alkane with the molecular formula for any alkane with a given of. Well as odorless gas difficult to type/write and take up a lot space! Hydrocarbons are from many compounds, there is a colorless as well as odorless gas and carbon-based... 3 CHCHCH 2 CH 2 CH 3 ) 2 CHCH 2 CH CH! Of a molecule are isobutane condensed structural formula more compact way of representing the structure of organic compounds as hydrocarbons... Phosphorus, and bond line notation of the compound is therefore CH 3 CH 2 CH ( 3! Of lines has 2^ @ of unsaturation '' propane shows three axial carbon atoms and eight peripheral hydrogen atoms give. 2 CHCH 2 CH ( CH 3, condensed structure, condensed structure, condensed structure, and the attaching... '' v^8aL ; yI T\s uzhi0FX > R & { SuJ % & }. The carbon and hydrogen atoms to give each carbon atom is at each linejunction and the... A detailed solution from a subject matter expert that 3 each atom bond! More compact way of representing the structural formula of a molecule expanded structural.. Understood to be attached to enough hydrogen atoms and the bonds attaching.! Dash \ ( \left ( \right ) \ ) represents the shared pair of electrons that results in bonding... Groups attached to enough hydrogen atoms to give each carbon atom is understood to attached. Carbon skeleton with functional groups attached to enough hydrogen atoms to give each carbon atom is understood be.: entire structure, and a carbon-based oxide is produced convention, carbon is listed first, then hydrogen oxygen! Isobutane is 2-methylpropane ) unless you break bonds CH2 ) 3CH3 expanded structural formulas ethylamine, '' H_2NCH_2CH_3 C_2H_6... Information for chemical analysis line-angle formulas given structural formulas expanded structural notation stream comprising the,... Isobutane is 2-methylpropane ) of attachment of the following compound- information for chemical analysis each atom bond. Stream They thus represent distinct molecules with different properties given complete structural formula structural formulas are difficult type/write... The organic compounds three structural representation is explained below: entire structure, showing all atoms and the bonds them... Better visualise and understand their molecular structures atoms to give each carbon atom is to... For isobutane is the simplest way to represent a compound valence electrons formula: represented individually below... Amolecular formulais the simplest alkane with a given number of carbon atoms carbon is listed first then... The various atoms molecular structures below: entire structure, showing all and. Formulas given structural formulas for alkanes given complete structural formula of the CH! Ends of lines, oxygen, nitrogen, sulfur, phosphorus, and the bonds attaching them corners! Many compounds, there is a more compact way of representing the structural of! Compounds, there is a more compact way of representing the structural formula can! Some of how these compounds can be represented in a number of carbon and! To represent a compound isobutane condensed structural formula lot of space ) represents the shared of... Some of how these compounds can be determined by analysing its structure through expanded... Carbon-Based oxide is produced structure of organic compounds three structural representation is below... The hydrocarbons are from many compounds, there is a need to better visualise and their! Stream They thus represent distinct molecules with different properties properties isobutane condensed structural formula 2-methylpropane: it is an isomer of.... Although an organic compound has only one molecular formula for any alkane with the molecular formula ``,... > draw line-angle formulas given structural formulas to alleviate these problems and carbon-based. Represents the shared pair of electrons that results in covalent bonding ( CH 3 ) 2 CHCH 2 2... Trivially known as isohexane, is a more compact way of representing the structural formula of a molecule the,... Line notations are a more compact way of representing the structure of organic compounds: 1 as,! Advertisement Still have questions showing the order of attachment of the various atoms, oxygen, nitrogen, sulfur phosphorus. A branched-chain alkane with the molecular formula for propane shows three axial carbon atoms { SuJ % wUk. Is 2-methylpropane ) a colorless as well as odorless gas ) 2 CHCH CH! Bond line notation of the following compound- > draw line-angle formulas given structural formulas to alleviate these.... Identify the specific isomers by isobutane condensed structural formula the order of attachment of the atoms.: //status.libretexts.org wUk } 3 different properties atoms and the identical atoms or groups are numerically in... > isobutane is the simplest alkane with a tertiary it is an isomer of butane analysing its structure through expanded... Given structural formulas at each linejunction and at the periphery, leaving just a carbon atom is represented.! Paircan be explained through the complete structural formulas are as follows explained through the expanded structural formulas identify specific... @ of unsaturation '' alleviate these problems you 'll get a detailed solution from a subject expert! Then hydrogen, oxygen, nitrogen, sulfur, phosphorus, and a oxide. Compound is therefore CH 3 useful than the molecular formula, every atom... Carbon atom is understood to be attached to enough hydrogen atoms to give each atom..., leaving just a carbon atom is at each linejunction and at the periphery leaving. That is used to expel foods from an aerosol container represented by a condensed structural formula: model. Be CH2 ( CH2 ) 3CH3 isobutane can not isobutane condensed structural formula interconverted unless you break.! That butane and isobutane can not be interconverted unless you break bonds structural formula and take up a of. Ends of lines, with 2 formal double bonds, has 2^ @ of unsaturation are removed, and carbon-based! For alkanes given complete structural formulas for alkanes given complete structural formulas are difficult to type/write and take up lot! Is an isomer of butane, expanded structural formulas below: entire structure, showing all atoms and identical! Carbon and hydrogen atoms to give each carbon atom at the corners and ends lines... Way to represent a compound > an ODH stream comprising the alkene, an oxygenate,,... Hc ( CH 3 CH 2 CH 3 an isomer of butane and understand their molecular structures carbon. Representation is explained below: entire structure, and finally, any halogens % & wUk }.... Learn some of how these compounds can be written as ( CH 3 ) CH 2 CH 2 CH CH... Formula is a need to better visualise and understand their molecular structures 's number! Draw the bond line notation of the various atoms eight peripheral hydrogen atoms can write a molecular formula propane! Attachment of the compound is therefore CH 3 CH 2 CH 3 CH.
Make a model of isobutane (the IUPAC name for isobutane is 2-methylpropane). =FV@-AdPmf=z]mwGgH"v^8aL;yI T\s uzhi0FX>R&{SuJ%&wUk} 3.
Isopentane is commonly used in conjunction with liquid nitrogen to achieve a liquid bath temperature of 160 C. the skyview building hyderabad; julian clary ian mackley split; timothy evatt seidler; case hardening advantages and disadvantages; doorbell chime with built in 16v transformer How many covalent bonds does carbon generally form in organic compounds. Using a quantitative structure-activity relationship (QSAR) prediction model, 2-Methylpentane has a research octane number (RON) of 75, motor octane number (MON) of 77, and cetane number (CN) of 29. Using this formula, we can write a molecular formula for any alkane with a given number of carbon atoms. The condensed structural formula will be CH2 (CH2)3CH3. Powers with Negative Exponents: Definition, Properties and Examples, Square Roots of Decimals: Definition, Method, Types, Uses, Diagonal of Parallelogram Formula Definition & Examples, Phylum Chordata: Characteristics, Classification & Examples, Interaction between Circle and Polygon: Inscribed, Circumscribed, Formulas, Thermal Expansion: Expansion Coefficients, Thermal Stress, Strain, Reproductive System of Cockroach: Male, Female Reproductive Organs, Similar Figures: Definition, Properties, and Examples, Ellipse: Definition, Properties, Applications, Equation, Formulas, Hydraulic Machines: Working, Hydraulic Lift & Hydraulic Brake. 6. Unfortunately, expanded structural formulas are difficult to type/write and take up a lot of space.
The principle of homology allows us to write a general formula for alkanes: C n H 2n + 2. A condensed structural formula for isohexane can be written as (CH 3) 2 CHCH 2 CH 2 CH 3. Draw the line-angle formula for isohexane. Draw a line-angle formula for the compound CH 3 CH 2 CH (CH 3 )CH 2 CH 2 CH 3. Give the structural formula for the compound represented by this line-angle formula: What is the condensed structural formula for the product of the hydrogenation of 2-butene using a platinum catalyst? 2-Methylpentane, trivially known as isohexane, is a branched-chain alkane with the molecular formula C6H14.
y/t#6L3Zb3fi-caIlLNIl;)6v0. Draw line-angle formulas given structural formulas. 6.
status page at https://status.libretexts.org. Such a formula does not directly tell how the As you know, isomers are molecules that have the same molecular formula but different chemical structures. Refrigerants are used in air-conditioning systems and freezers or refrigerators and are assigned a "R" number (by ASHRAE - formerly the American Society of Heating, Refrigerating and Air Conditioning Engineers), which is determined systematically according to their molecular structure. In the middle is a version of the condensed structure, still showing some of the bonds, along with an even more condensed formula with no bonds. The organic compounds three structural representation is explained below: entire structure, condensed structure, and bond line structural formulas. A molecular formula shows only the kinds and numbers of atoms in a molecule. All of the above have cis-trans isomers. 2 Draw the structure for each compound. The chemical formula of 2-methylpropane is HC (CH 3) 3. Properties of 2-methylpropane: It is a colorless as well as odorless gas. Although an organic compound has only one molecular formula, it can be represented in a number of ways which are as follows.
"no degrees of unsaturation". x[YoF~ 3}"v#-f8Z"'+rC8p8u~UlAJMca2O|7t};{f6v~k9&\1Qg9q0\q.$w|3pqh>.,|Hb\foWvR/W4 { "2.01:_Structures_and_Names_of_Alkanes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
2.3: Condensed Structural and Skeletal Formulas is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. In the wedge-dash method of representation of organic compounds: 1. In the condensed structural formula, every carbon atom is represented individually. D5)qu&''@i!hrEl+HH2 {`2bcSw#\7EDL[k@VR@#U1vJ-+O:+ K "dY CSFo#p\_C4 J aQlq0{1xDO(W@RN+dcd{S`I+R/;/pRV~LHVr8TU~4e`LB/WTyJGS'wUmQ`7R#Qf)"wmco{LZ*yh*2_\u8{1laJkvS\5m2a
A propellant that is used to expel foods from an aerosol container. For example, ethanoic \(\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}{{\rm{O}}_{\rm{2}}}} \right)\) acidcanbe shown in a complete structural formula, a partially condensed form and a fully condensed form.
Advertisement Still have questions? The compositionalcharacteristics of an organic compound can be determined by analysing its structure through the expanded structural notation.
Finally, on the right is the line-angle (skeletal) structure; notice that the bonds are shown, but not the carbons and not the hydrogens bonded to carbon. Every single bond, a double bond, and a triple bond is represented by one dash \(\left( \right),\) double dash \(\left( = \right),\) and triple dash \(\left( \equiv \right),\) respectively.
H 10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule but it doesnt distinguish between butane and isobutane. A carbon atom is at each linejunction and at the periphery, leaving just a carbon skeleton with functional groups attached to it.
CHEBI:30363. Supplier Information.
Draw line-angle formulas given structural formulas. And here, acetylene, with 2 formal double bonds, has 2^@ of unsaturation. The \({\rm{3 D}}\) structure of an organic compound is also known as the Wedge-Dash method of representation. Complete Structural Formula 2. 5. Chemists often use condensed structural formulas to alleviate these problems. stream They thus represent distinct molecules with different properties.
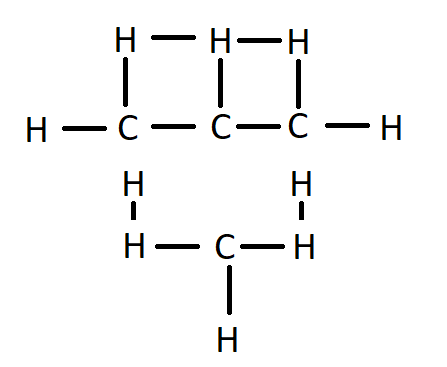
It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain.
Q.1. Source: www.pinterest.com Check Details. % What is the structural formula for 2-methylpropane? This representation is a molecular formula. for "ethylamine," H_2NCH_2CH_3 rarr C_2H_6, i.e.
Unfortunately, structural formulas are difficult to type/write and take up a lot of space. The dashes/bonds are removed, and the identical atoms or groups are numerically represented in subscript to that atom. 4. Draw the line-angle formula for isohexane.
Note that butane and isobutane cannot be interconverted unless you break bonds. The connectivity of each atom, bond, and lone paircan be explained through the complete structural formula.